Research
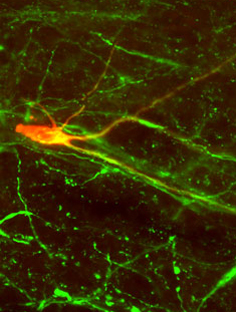
Biocytin-filled neuron co-labeled with tryptophan hydroxylase in the RVM
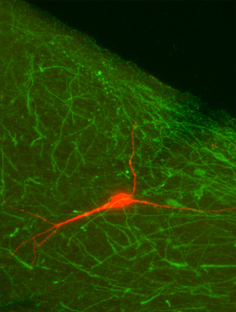
Biocytin-filled neuron not co-labeled with tryptophan hydroxylase in the ventrolateral PAG
Relieving pain is a major challenge for clinicians, which highlights the continuing need to identify novel targets for antinociceptive agents. Analgesia is an important effect of nicotinic acetylcholine receptors (nAChRs). The identification of selective agonists of these receptors with analgesic efficacy similar to morphine has generated considerable interest and excitement. Activation of both supraspinal, as well as spinal nAChRs likely contribute to this antinociception. Consistent with this idea, activation of α4β2 subtype nAChRs in the rostral ventromedial medulla (RVM) inhibits transmission of nociceptive signals. Unfortunately, the nicotinic analgesic compounds that have been tested in humans produce negative side effects that have precluded their clinical use. Despite these advances, the underlying cellular mechanisms that mediate both the antinociception and the unfortunate side effects are poorly understood. This highlights the need for a thorough investigation of the impact of nAChRs on descending antinociceptive pathways. The RVM receives afferent input from the periaqueductal grey (PAG), another important relay in the descending control of pain. Anatomical and in vivo pharmacololgical studies suggest that that nAChRs in PAG may also contribute to antinociception, but this has not been investigated. Our hypothesis is that distinct subtypes of nAChRs in the PAG and RVM contribute to descending analgesia. A systematic investigation of nAChR modulation of descending pain control circuitry is a critical step toward the identification of novel therapeutics for the treatment of acute and chronic pain.
- J.R. Genzen, W. Van Cleve and D.S. McGehee. (2001) Dorsal root ganglion neurons express multiple nicotinic receptor subtypes. Journal of Neurophysiology 86(4): 1773-82.
- J.R. Genzen and D.S. McGehee. (2003) Short and Long-term enhancement of excitatory transmission in the spinal cord dorsal horn by nicotinic acetylcholine receptors. Proceedings of the National Academy of Sciences 100(11):6807-6812.
- J.R. Genzen & D.S. McGehee. (2005) Nicotinic modulation of GABAergic synaptic transmission in the spinal cord dorsal horn. Brain Research 1031(2):229-37.